Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hashimoto, Shoji*; Tanaka, Taku*; Komatsu, Masabumi*; Gonze, M.-A.*; Sakashita, Wataru*; Kurikami, Hiroshi; Nishina, Kazuya*; Ota, Masakazu; Ohashi, Shinta*; Calmon, P.*; et al.
Journal of Environmental Radioactivity, 238-239, p.106721_1 - 106721_10, 2021/11
Times Cited Count:11 Percentile:56.59(Environmental Sciences)This study was aimed at analysing performance of models for radiocesium migration mainly in evergreen coniferous forest in Fukushima, by inter-comparison between models of several research teams. The exercise included two scenarios of countermeasures against the contamination, namely removal of soil surface litter and forest renewal, and a specific konara oak forest scenario in addition to the evergreen forest scenario. All the models reproduced trend of time evolution of radiocesium inventories and concentrations in each of the components in forest such as leaf and organic soil layer. However, the variations between models enlarged in long-term predictions over 50 years after the fallout, meaning continuous field monitoring and model verification/validation is necessary.
Moribayashi, Kengo
Physica Scripta, 90(5), p.054013_1 - 054013_5, 2015/05
Times Cited Count:5 Percentile:40.49(Physics, Multidisciplinary)Arthur, R. C,*; Savage, D.*; Sasamoto, Hiroshi; Shibata, Masahiro; Yui, Mikazu
JNC TN8400 2000-005, 61 Pages, 2000/01
Kinetic data, including rate constants, reaction orders and activation energies, are compiled for 34 hydrolysis reactions involving feldspars, sheet silicates, zeolites, oxides, pyroxenes and amphiboles, and for similar reactions involving calcite and pyrite. The data are compatible with a rate law consistent with surface reaction control and transition-state theoly, which is incorporated in the geochemieal software package EQ3/6 and GWB. Kinetic data for the reactions noted above are strictly compatible with the transition-state rate law only under far-from-equilibrium conditions. It is possiblethat the data are conceptually consistent with this rate law under both far-from-equilibrium and near-to-equilibrium conditions, but this should be confirmed whenever possible through analysis of original experimental results, Due to limitations in the availability of kinetic data for mineral-water reactions, and in order to simplify evaluations of geochemical models of groundwater evolution, it is convenient to assume local-equilibrium in such models whenever possible. To assess whether this assumption is reasonable, a modeling approach accounting for coupled fluid flow and water-rock interaction is described that can be used to estimate spatial and temporal scale of local equiliblium. The approach is demonstrated for conditions involving groundwater flow in fractures at JNC's Kamaishi in-situ tests site, and is also used to estimate the travel time necessary for oxidizing surface waters to migrate to the level of a HLW repository in crystalline rock. The question of whether local equilibrium is a reasonable assumption must be addressed using an appropriate modeling approach. To be appropriate for conditions at the Kamaishi site using the modeling approach noted above, the fracture fill must closely approximate a porous medium, groundwater flow must be purely advective and diffusion of solutes across the fracture-host rock boundary must not occur. Moreover, the ...
Savage, D.*; Lemke, K.*; Sasamoto, Hiroshi; Shibata, Masahiro; Arthur, R. C,*; Yui, Mikazu
JNC TN8400 2000-004, 30 Pages, 2000/01
Modeling approaches that have been proposed for cement-water system are reviewed in this report, and relevant supporting thsrmodynamic data are compiled. The thermodynamic data include standard molal thermodynamic properties of minerals and related compounds comprising cements, and equilibrium constants for associated hydrolysis reactions. Similar data for minerals that are stable in hyperalkaline geologic environments (e.g., zeolites) are also included because these minerals could be formed as hyperalkaline fluids emanating from cementitious matelials in a repository for radioactive wastes interact with the surrounding host rock. Standard molal properties (i.e., standard molal Gibbs free energies and enthalpies of formation and standard molal entropies), and/or equilibrium constants for associated hydrolysis reactions, are included for. (1)cement minerals and related compounds (Reardon, 1992; Glasser et al., 1999) (2)calcium-silicate hydrate minerals (Sarkar et al., 1982), and (3)zeolites (calorimetric and estimated values from various sources) All these data are accepted at face value, and it is therefore cautioned that the data, considered as a whole, may not be internally consistent. It is also important to note that the accuracy of these data have not been evaluated in the present study. Several models appropriate for cement-water systems have been proposed in recent years. Most are similar in the sense that they represent empirical fits to laboratory data for the CSH gel-water system, and therefore not thermodynamically defensible. An alternative modeling approach based on thermodynamic principles of solid-solution behavior appropriate for CSH gel has recently been proposed, however. It is reviewed in the present study, and evaluated in relation to experimental results obtained by JNC on cement-water interactions. The solid-solution model is based upon a thermodynamically- and structually-justifiable description of CSH gel in terms of a non-ideal ...
Sasamoto, Hiroshi; Yui, Mikazu; Randolph C Arthu*
JNC TN8400 99-074, 84 Pages, 1999/12
Hydrochemical investigation of Tertiary sedimentary rocks at JNC's Tono in-situ tests site indicate the groundwaters are: (1)meteoric in origin, (2)chemically reducing at depths greater than a few tens of meters in the sedimentary rock, (3)relatively old [carbon-14 ages of groundwaters collected from the lower part of the sedimentary sequence range from 13,000 to 15,000 years BP (before present)] (4)Ca-Na-HCO type solutions near the surface, changing to Na-HCO
type solutions near the surface, changing to Na-HCO type groundwaters with increasing depth. The chemical evolution of the groundwaters is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, Al, carbonate and sulfate) if the following assumptions are adopted: (1)CO
type groundwaters with increasing depth. The chemical evolution of the groundwaters is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, Al, carbonate and sulfate) if the following assumptions are adopted: (1)CO concentration in the gas phase contacting pore solutions in the overlying soil zone = 10
concentration in the gas phase contacting pore solutions in the overlying soil zone = 10 bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), muscovite (K) and pyrite (Eh and sulfate). It is noted, however, that the available field data may not be sufficient to adequately constrain parameters in the groundwater evolution model. In particular, more detailed information characterizing certain site properties (e.g., the actual mineralogy of "plagioclase", "clay" and "zeolite") are needed to improve the model. Alternative conceptual models of key reactions may also be necessary. For this reason, a model that accounts for ion-exchange reactions among clay minerals, and which is based on the results of laboratory experiments, has also been evaluated in the present study. Further improvements of model considering ion-exchange reactions are needed in future, however.
bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), muscovite (K) and pyrite (Eh and sulfate). It is noted, however, that the available field data may not be sufficient to adequately constrain parameters in the groundwater evolution model. In particular, more detailed information characterizing certain site properties (e.g., the actual mineralogy of "plagioclase", "clay" and "zeolite") are needed to improve the model. Alternative conceptual models of key reactions may also be necessary. For this reason, a model that accounts for ion-exchange reactions among clay minerals, and which is based on the results of laboratory experiments, has also been evaluated in the present study. Further improvements of model considering ion-exchange reactions are needed in future, however.
*; Sato, Haruo; *
JNC TN8400 99-059, 59 Pages, 1999/10
Organic acids in groundwater are considered to form complexes and increase the solubility of radionuclides released from vitrified waste in a high-level radioactive waste (HLW) repository. To ivestigate whether the solubility of samarium (Sm) is influenced by organic substances, we measured Sm solubility in the presence of different organic substances and compared those values with results from thermodynamic predictions. Humic acid (Aldrich) is commercially available and soluble organic matter originated from bentonite were used as organic substances in this study. Consequently, the solubility of Sm showed a tendency to apparently increase with icreasing the concentration of humic acid, but in the presence of carbonate, thermodynamic predictions suggested that the dominant species are carbonate complexes and that the effect of organic substances are less than that of carbonate. Based on total organic carbon (TOC), the increase of Sm solubility measured with humic acid (Aldrich) was more significant than that in the case with soluble organic matter originated from bentonite. Since bentonite is presumed to include also simple organic matters of which stability constant for forming complexes is low, the effect of soluble organic matter originated from bentonite on the solubility of Sm is eonsidered to be less effective than that of humic acid (Aldrieh). Experimental values were compared with model prediction, propsed by Kim, based on data measured in a low pH region. Tentatively we calculated the increase in Sm solubility assuming complexation with humic acid. Trial calculations were carried out on the premise that the complexation reaction of metal ion with humic acid is based on neutralization process by 1-1 complexation. In this process, it was assumed that one metal ion coordinates with one unit of complexation sites which number of proton exchange sites is equal to ionic charge. Consequently, Kim's model indicated that carbonate complexes should be dominant ...
Sasamoto, Hiroshi; Yui, Mikazu; Arthur, R. C,*
JNC TN8400 99-033, 153 Pages, 1999/07
The results of hydrochemical investigations of groundwaters in the Kurihashi granodiorite at JNC's Kamaishi in-situ tests site indicate that these solutions are: (1)meteoric in origin, (2)chemically reducing (at depths greater than a few hundreds meters), (3)relatively young [residence times in the Kurihashi granodiorite generally less than about 40 years, but groundwaters older than several thousand years BP (before present) are also indicated by preliminary carbon-14 dating of samples obtained from the KH-1 borehole], (4)Ca-HCO type solutions near the surface, changing to Na-HCO
type solutions near the surface, changing to Na-HCO type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO
type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO concentration in the gas phase contacting pore solutions in the overlying soil zone = 10
concentration in the gas phase contacting pore solutions in the overlying soil zone = 10 bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.
bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.
Yui, Mikazu; Sasamoto, Hiroshi; Randolph C Arthu*
JNC TN8400 99-030, 201 Pages, 1999/07
According to the Japanese program for research and development of high level radioactive waste (HLW) disposal defined by Atomic Energy Commission (AEC), the second progress report (i.e., H-12 report) for performance assessment (PA) of HLW disposal is to be published by the Japan Nuclear Cycle Development Institute (JNC) and submitted to the Japanese government before the year 2,000 (AEC, 1997). This report presents the establishment of generic groundwater chemical compositions for the PA supporting the H-12 report. The following five hypothetical groundwaters are categorized for PA based on the results of the first progress report (i.e., H-3 report) and binaly statistical analyses of the screened groundwater dataset: (1)FRHP(Fresh-Reducing-High-pH) groundwater (2)FRLP(Fresh-Reducing-Low-pH) groundwater (3)SRHP(Saline-Reducing-High-pH) groundwater (4)SRLP(Saline-Reducing-Low-pH) groundwater (5)MRNP(Mixing-Reducing-Neutral-pH) groundwater. In order to define representative groundwater compositions for the PA for the H-12 report, JNC has established the representativeness of the above five hypothetical groundwaters by considering the results of multivariate statistical analyses, data reliability, evidence for geochemical controls on groundwater chemistry and exclusion criteria for potential repository sites in Japan. As a result, the following hypothetical reference groundwaters are selected for the performance assessment analysis in H-12 report, respectively: (1)Reference Case groundwater: FRHP groundwater, and (2)Alternative Geological Environment Case groundwater: SRHP groundwater. In addition, JNC has consulted with overseas experts on the concepts used in groundwater evolution modeling. This modeling effort has focussed on simulating equilibrium water-rock interactions to predict groundwater compositions resulting from reactions between initial water compositions and rock mineral assemblages. These discussions have centered on recommendations for developing ...
G M N BASTON*; J A BERRY*; M BROWNSWORD*; D J LLETT*; C M LINKLATER*; S W SWANTON*; Tweed, C. J.*
JNC TJ8400 99-078, 72 Pages, 1999/03
A desk study has been carried out to establish the feasibility of measuring the oxidation state of plutonium under near-neutral strongly-reducing conditions. X-ray absorbance spectroscopy appears to be capable of establishing the oxidation state of plutonium sorbed on a suitable substrate. An experimental and modelling investigation has been performed to study the sorption of plutonium onto basalt, mudstone and sandstone under strongly-reducing conditions at three concentrations of carbonate. Appropriate synthetic rock-equilibrated de-ionised water and seawater were used. A model has been developed to describe the sorption of plutonium onto basalt, mudstone and sandstone in de-ionised water and seawater. Predicted R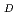 values are generally in good agreement with the observed experimental measurements. The model is based on sorption of plutonium(III) species and assumes iron oxide is the dominant sorbing phase.
values are generally in good agreement with the observed experimental measurements. The model is based on sorption of plutonium(III) species and assumes iron oxide is the dominant sorbing phase.
Ueta, Shinzo*
PNC TJ1211 98-002, 46 Pages, 1998/02
None
Ueta, Shinzo*
PNC TJ1211 98-001, 824 Pages, 1998/02
None
; Ohno, Shuji; Miyake, Osamu; ; Seino, Hiroshi
PNC TN9520 97-001, 185 Pages, 1997/12
ASSCOPS(Analysis of Simultaneous Sodium Combustion in Pool and Spray) has been developed for analyses of thermal consequences of sodium leak and fire accidents in LMFBRs. This report presents a description of the computational models, input, and output as the user's manual of ASSCOPS version 2.0. ASSCOPS is an integrated code based on the sodium pool fire code SOFIRE II developed by the Atomics International Division of Rockwell International, and the sodium spray fire code SPRAY developed by the Hanford Engineering Development Laboratoly in the U.S. The experimental studies conducted at PNC have been reflected in the ASSCOPS improvement. The users of ASSCOPS need to specify the sodium leak conditions (leak flow rate and temperature, etc.), the cell geometries (volume and structure surface area and thickness, etc.), and the atmospheric initial conditions, such as gas temperature, pressure, and gas composition. ASSCOPS calculates the time histories of atmospheric pressure and temperature changes along with those of the structural temperatures.